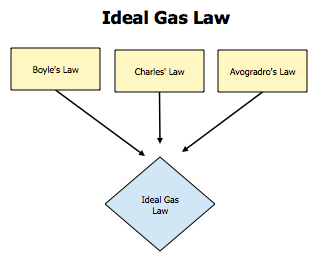
Last week we began our gas law unit. We started with ideal gasses and how they behave. We worked a lot with the ideal gas law, PV=nRT where P= pressure in atmospheres, V=volume in litres, n=number of moles, R=.0821 or the universal gas constant, and T=temperature in degrees kelvin. Derived from this ideal gas law equation are five other gas laws, named after the scientists who contributed to them. Gay-Lussac's law states that pressure and temperature are directly related through the relationship P1/T1=P2/T2. Charles' law states that volume and temperature are directly related through the relationship V1/T1=V2/T2. In Boyle's law, volume and pressure are inversely related, P1V1=P2V2. Dalton's law says that you can add partial pressures to get the total pressure. Avogadro's law states that volume and number of particles are directly related, V1/N1=V2N2. If you combine Gay-Lussac's Charles' and Boyle's laws, then you get the combined gas law, (P1V1/T1=P2V2/T2) which is good to remember because there's no need to remember the individual other laws, just cover up the constant and you have the equation you need.
I understand ideal gasses pretty well, sometimes the math can be a bit confusing, but only the stoich parts.
Real gasses take into account the attractiveness of particles to each other, and also their volume. If the particle is bigger, it will make the total volume bigger and also it will be more polarizable, making the pressure go down due to the particles pulling themselves from the walls. The volume for ideal gasses is adjusted up by a and the pressure for ideal gasses is adjusted down by b due to these phenomena.
I understand gas laws pretty well, but not perfectly. I still have work to do. My major problem with any test has seemed to have been the math part, so I need to get some extra practice with that tonight and tomorrow.